Fluorine The Most Reactive Element in Periodic Table Owlcation
Name: Fluorine Symbol: F Atomic Number: 9 Atomic Mass: 18.998404 amu Melting Point:-219.62 °C (53.530006 K, -363.31598 °F) Boiling Point:-188.14 °C (85.01 K, -306.652 °F) Number of Protons/Electrons: 9 Number of Neutrons: 10 Classification: Halogen Crystal Structure: Cubic Density @ 293 K: 1.696 g/cm 3 Color: Greenish Atomic Structure
13+ Diagram Of Fluorine ArmaanDallas
1 Ry = e4me 8ϵ2 0h2 = 2.18 × 10 − 18 J. and this simplifies the allowed energies predicted by the Bohr model (Equation 7.4.11) as. En = − (2.18 × 10 − 18)Z2 n2 J = − Z2 n2 Ry. Hence, the energy of the electron in an atom also is quantized. Equation 7.4.12 gives the energies of the electronic states of the hydrogen atom.

Fluorine Atom Diagram
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.

Fileelectronformula N2svg Wikimedia Commons
Bohr Diagrams. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure \(\PageIndex{2}\) contrast the Bohr diagrams for lithium, fluorine and aluminum atoms.

Bohr Model Of Fluorine
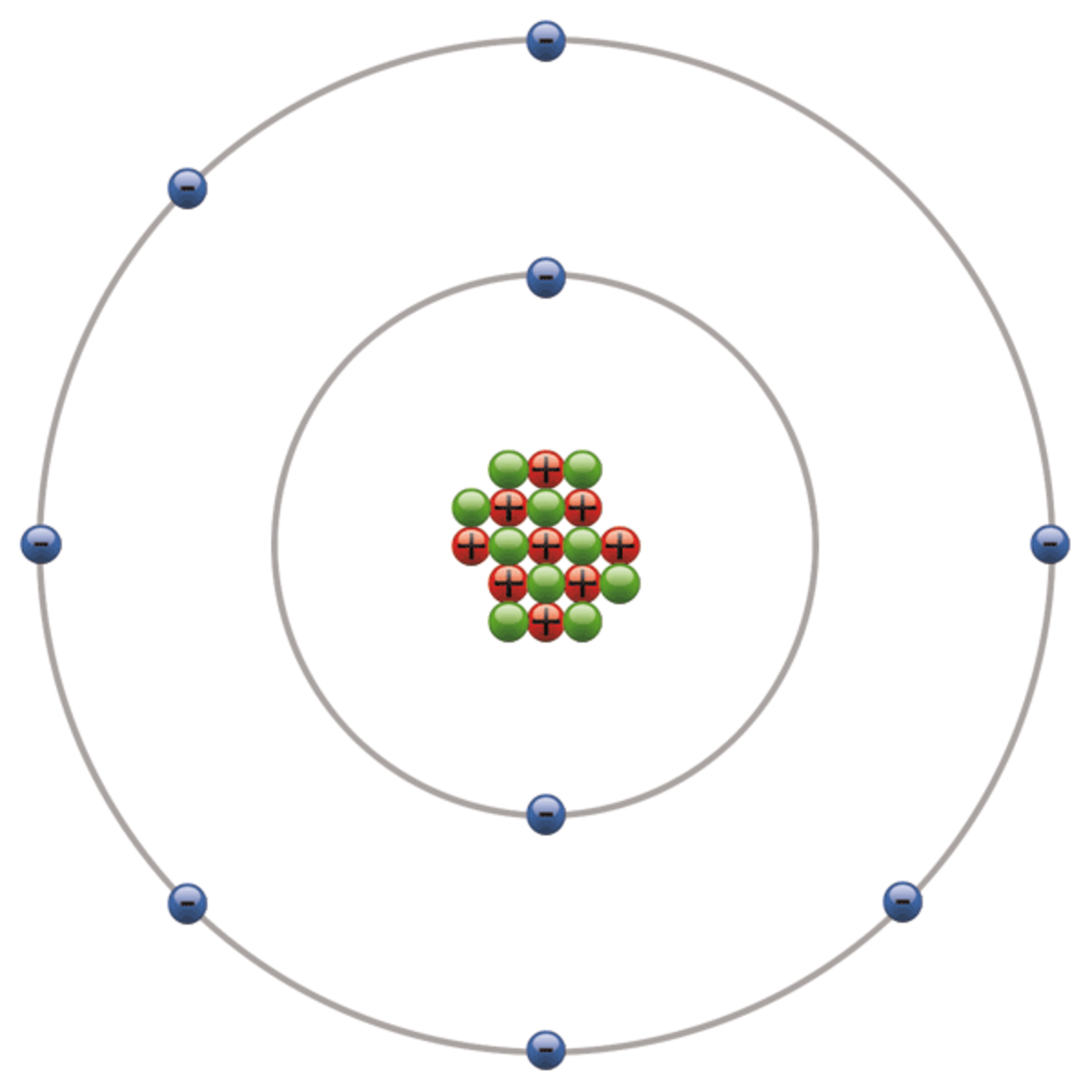
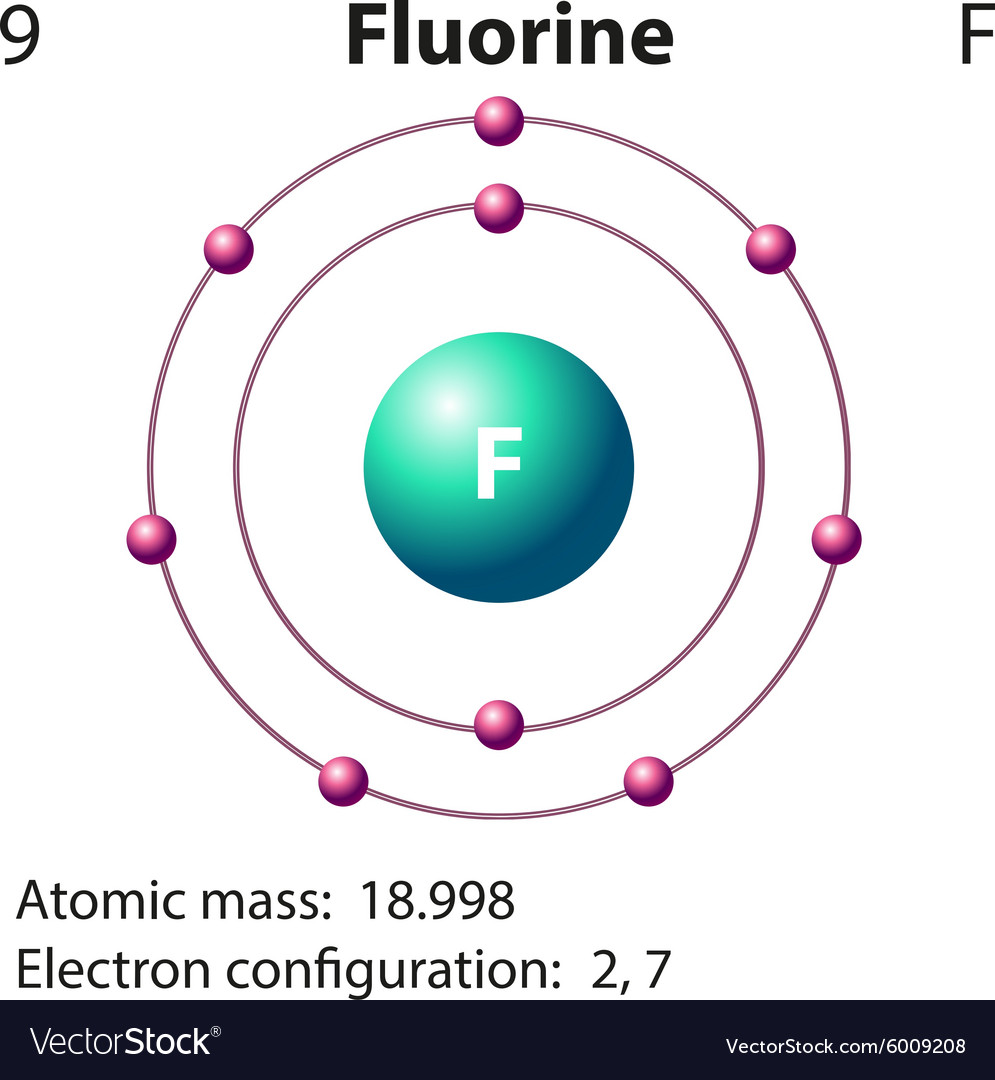
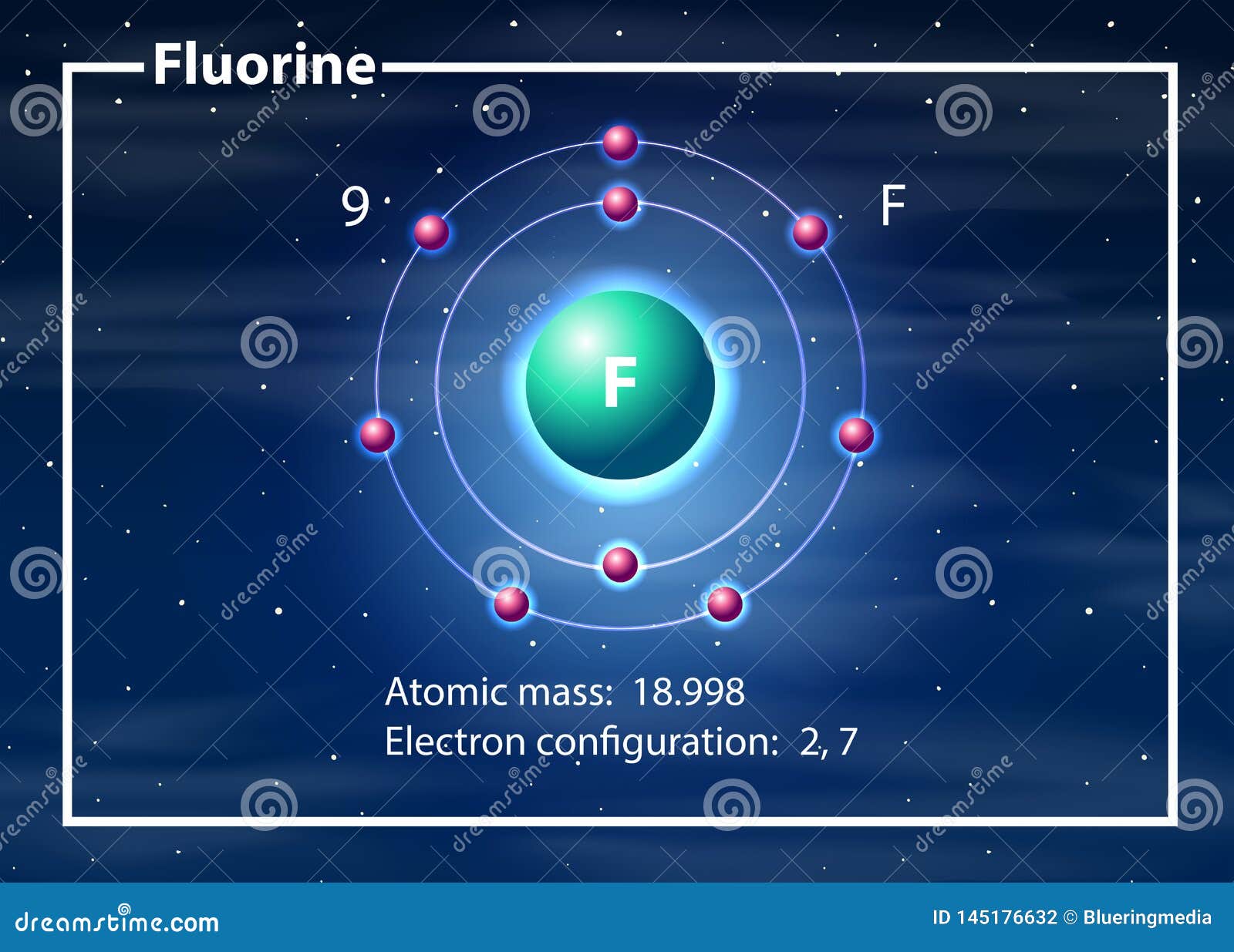
This is the Bohr-Rutherford Diagram for Fluorine (Atomic Number 9). Fluorine is the ninth element of the Periodic Table. As shown, Fluorine has nine protons (i.e., 9p) and ten neutrons (i.e., 10n). Thus, its Atomic Mass is 19. Fluorine also has nine total electrons -- two electrons in Orbit 1, seven electrons in Orbit 2.
Fluorine Atom Bohr Model With Proton, Neutron And Electron RoyaltyFree
By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior.Devised by Russian chemist Dmitri Mendeleev (1834-1907) in 1869, the table places elements into columns—groups—and rows—periods—that share certain properties.These properties determine an element's physical state at room temperature—gas, solid, or liquid.

Fluorine Atom Diagram
Fluorine has 2 electrons in its first shell and 7 in its second.Check me out: http://www.chemistnate.com

Fluorine Electron Dot Diagram
In this video we'll look at the atomic structure and Bohr model for the Fluorine atom (F). We'll use a Bohr diagram to visually represent where the electrons.
Fluorine Bohr Diagram
On the far left of Figure 3.6.1 3.6. 1 are the highest energy electromagnetic waves. These are called gamma rays and can be quite dangerous, in large numbers, to living systems. The next lower energy form of electromagnetic waves are called x-rays. Most of you are familiar with the penetration abilities of these waves.
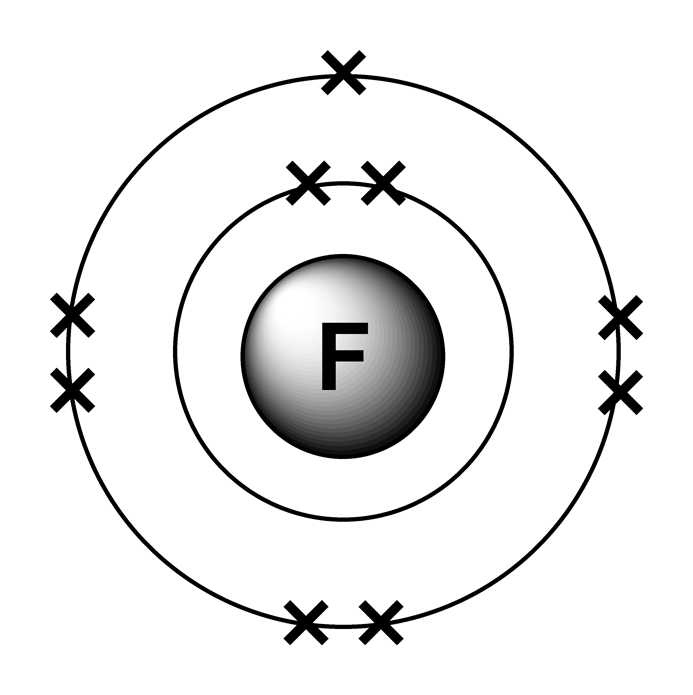
Fluorine Electron Dot Structure My XXX Hot Girl
Fluorine is a Group 7 element, on the Periodic Table, with an atomic number of 9. About Fluorine Molecular Structure Fluorine has the chemical formula F 2. Atomic Structure Fluorine as 9 protons and 10 neutrons in its nucleus giving it an Atomic Number of 9 and an atomic mass of 19. An atom of Fluorine is missing one electron from having a full.
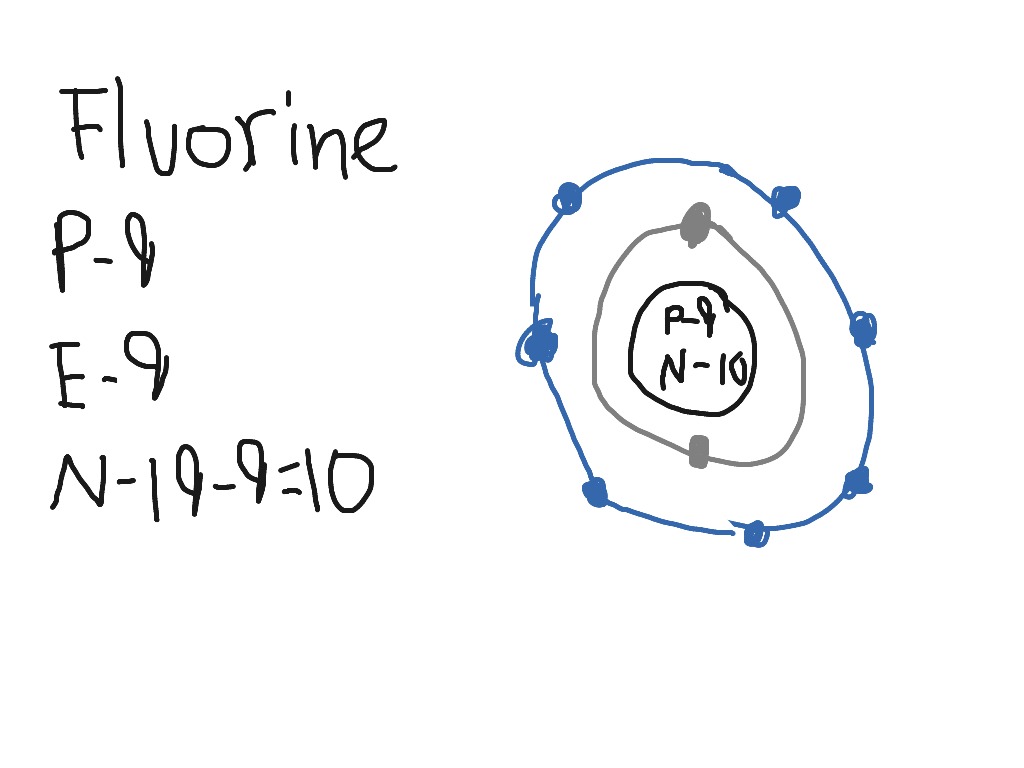
Bohr Diagram For Fluorine General Wiring Diagram
For more information and resources, please visit: https://sites.google.com/tdsb.on.ca/htc-meyer/home/9-Science/9-chemistry

Fluorine Bohr Diagram
Bohr diagram is very interesting and easy to draw. Here, we will draw the Bohr diagram of the Fluorine atom with some simple steps. Steps to draw the Bohr Model of Fluorine atom 1. Find the number of protons, electrons, and neutrons in the Fluorine atom

Fluorine Diagram
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ).

How Many Electrons Does Fluorine Have five empat
Bohr diagrams for hydrogen, helium, lithium, carbon, fluorine, neon, sodium, silicon, chlorine, and argon. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration.

What is the Bohr model for fluorine? Quizlet
Bohr Model of all Elements (Diagrams + Chart) March 23, 2023 by Jay Bohr model of all Elements is mentioned in the chart below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1).
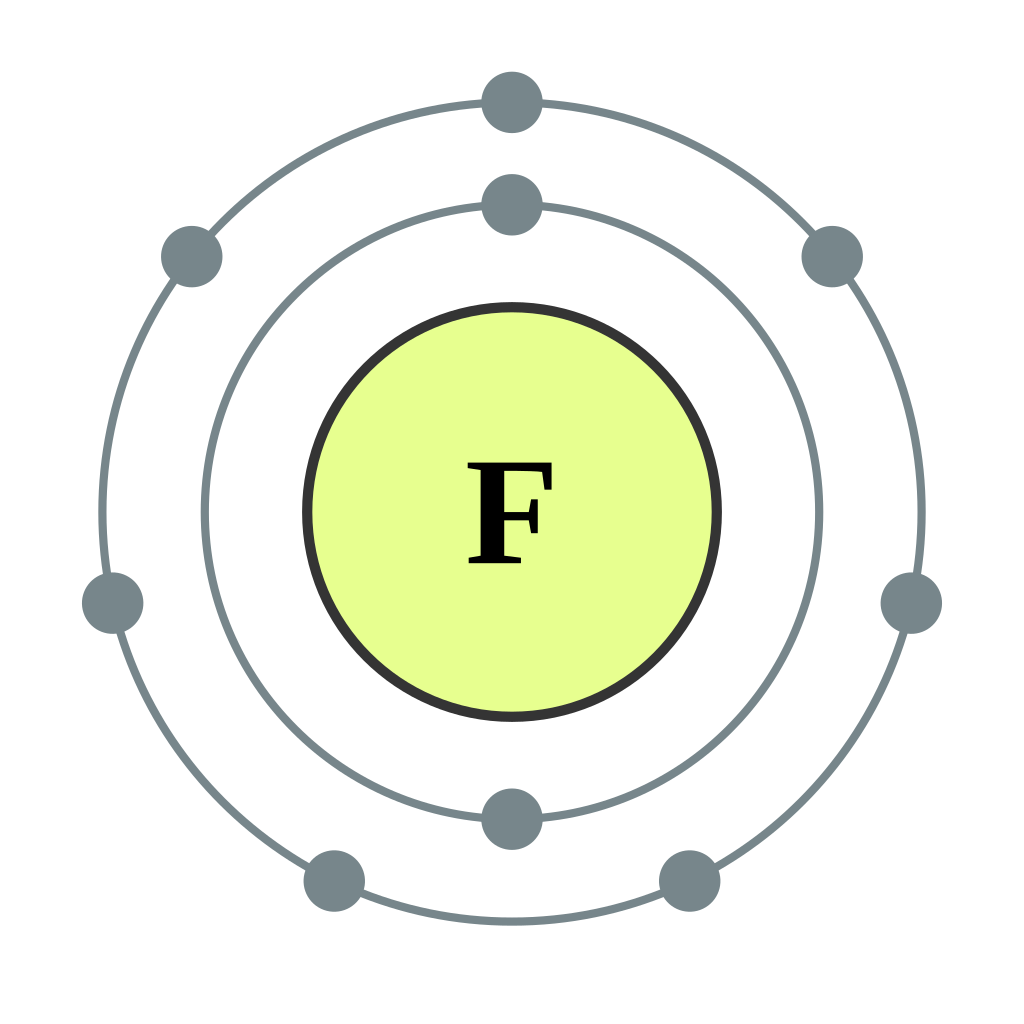
Bohr Diagram For Fluorine Photos Cantik
Podcasts Produced by The Naked Scientists. Periodic Table of Videos Created by video journalist Brady Haran working with chemists at The University of Nottingham. Element Fluorine (F), Group 17, Atomic Number 9, p-block, Mass 18.998. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.